Resource Centre
ASLM’s Resource Center is designed to help you keep up with the latest tool kits, regulations, guides and other information published by ASLM, partners, and global health regulatory bodies. Use the filters above to locate the information you need based on the ASLM project, resource type, or topic of interest. To find a specific resource, enter the resource name in the search bar. You may also use the search bar to enter key words related to the resources of your interest.
The February LabCoP ECHO Session focused on low-level viremia during anti-retroviral therapy. Progress in research on HIV/AIDS, the implementation of interventions to support prevention, case identification and availing lifesaving antiretroviral treatment (ART) have been generally successful. Despite these strides, up to 39 Million people globally were living with HIV and about 630,000 died from AIDS-related… Read More
In April 2023, ASLM’s Laboratory Directors Forum convened a high-level meeting on diagnostic integration and laboratory systems strengthening in Nairobi, Kenya. The meeting brought together 97 attendees, including 53 in-person and 44 virtual participants, consisting of laboratory directors, HIV and tuberculosis (TB) programme managers from 15 countries, global health experts, funders, and collaborating partners. This… Read More
The July 2023 LabCoP ECHO session, jointly organized by ASLM and Cepheid, focused on point-of-care (POC) HIV viral load (VL) at delivery to facilitate early infant diagnosis (EID) and maternal adherence in Uganda. In this session, we discuss results of a randomized trial focussing on the use of POC testing for EID and VL to… Read More
The Laboratory System’s Strengthening Community of Practice (LabCoP) 6th Annual Meeting report presents key summaries, proposed actions and recommendations from the 2022 annual meeting. Held in Cape Town, South Africa from 11-13 October 2022, this hybrid in-person and virtual meeting brought together 19 country teams, funding agencies, ASLM collaborating partners and the LabCoP Management Team…. Read More
LabCoP’s March 2022 ECHO session focused on generating demand for HIV viral load (VL) testing through targeted communications campaigns. For people living with HIV and on antiretroviral treatment, taking a VL test is essential in monitoring if the treatment is working. The World Health Organization recommends at least one annual VL test. Yet, knowledge around… Read More
The HIV Laboratory Waste Cost Assessment Framework (WCAF) is a tool to support laboratories with a standardised format for forecasting waste disposal costs for HIV viral load (VL) and early infant diagnosis (EID) to country level program managers for country operational plan (COP) planning. This tool was co-developed by US CDC and Roche Diagnostics under… Read More
On 4 November 2021, ASLM`s LabCoP monitoring and evaluation (M&E) sub-community of practice, in collaboration with the World Health Organization, convened a session focusing on monitoring country level viral load (VL) programs. Countries are putting in place robust systems to evaluate HIV VL programs to determine if set objectives, desired outcomes and impactful results are… Read More
In September 2021 the World Health Organization released updated guidance on maintaining essential health services during COVID-19, which provides direction on modifications and specific measures for safe delivery of HIV services, and considerations for transition towards restoration and recovery. Surveys of 101 countries have revealed substantial disruptions across all major health areas with up to… Read More
Low- and middle-income countries (LMIC) currently experience low testing coverage in the face of increasing demand to meet the needs of millions of people living with HIV, TB, HPV, hepatitis and other diseases. Therefore, innovative solutions are urgently needed to enable a robust, efficiently utilised testing capacity, and a healthy and secure market with competitive… Read More
LabCoP’s August 2021 ECHO session focused on sharing progress in HIV viral load (VL) monitoring among patients on antiretroviral therapy (ART) from eight sub-Saharan Africa countries between 2013 and 2018. Monitoring of VL suppression at individual and population level is necessary to ensure achievement of global epidemic control. Despite significant challenges that hinder uptake in… Read More
LabCoP’s June 2021 ECHO session focussed on generating demand for HIV viral load (VL) testing through targeted communications campaigns. WHO recommends at least one annual VL test for each person on Highly Active Antiretroviral Therapy (HAART) as this is essential in monitoring if treatment is working. Generally, knowledge around the importance of VL tests as… Read More
In June the ASLM LabCoP monitoring and evaluation (M&E) Sub-CoP held an ECHO session on viral load (VL) testing data quality assessment. The presentation provided an overview of the 2020 WHO-UNAIDS-PEPFAR-Global Fund module for assessing and strengthening the quality of VL testing data within HIV programmes and patient monitoring systems. Kenya shared their country’s VL… Read More
These guidelines, published by the World Health Organization, provide new and updated recommendations and good practice statements in the following areas: starting antiretroviral therapy (ART), including initiating treatment outside the clinic and support for same-day ART start; frequency of clinical visits and ART refills; measuring adherence; tracing and re-engagement in care for all populations; psychosocial… Read More
In May 2021 the ASLM LabCoP monitoring and evaluation (M&E) Sub-CoP held a session on data management, dashboards and connectivity solutions for HIV viral load (VL) and early infant diagnosis (EID). Presentations were made by Yonatan Gossaye Dessalegn, Senior Associate Software Developer on Clinton Health Access Initiative (CHAI) Global Laboratory Services Team; Kalechristos Abebe, IT… Read More
On 22 April 2021 ASLM’s LabCoP M&E Sub-community of practice held a south-to-south learning session during which the Uganda country team shared their experience in rolling out monitoring and evaluation (M&E) systems for viral load (VL) including virtual dashboards that allow for performance tracking at various administrative levels. The session covered key aspects of data… Read More
On 1 April 2021, ASLM`s LabCoP M&E Sub-CoP, in collaboration with U.S. Centers for Disease Control and Prevention (CDC), Division of Global HIV and Tuberculosis (DGHT) convened a session focusing on indicators for scale-up of viral load (VL) testing and program outcomes. Presentations were made by Nadia Solehdin, an epidemiologist, and Kat Sisler, an evaluation… Read More
This presentation by the Clinton Health Access Initiative outlines the steps of an integration implementation framework for tuberculosis (TB) and HIV diagnostics, and related diseases, top tips and caveats (what could go wrong and lessons learned) from pilot countries on each step and area of implementation. It provides a deeper understanding of activities required for… Read More
This presentation by the Clinton Health Access Initiative outlines the need for point-of-care (POC) testing, how integrated testing improves access to POC, the benefits of integration, and considerations and implementation framework for integrated testing.
The GX Capacity Utilisation Analysis Tool is an Excel-based tool to assess the capacity utilisation of GeneXpert platforms for integrating tuberculosis (TB), HIV, HCV and HPV testing. It has been used to identify platforms running with full-capacity and with un-used capacity to inform integration decisions in Cameroon, Ethiopia, Indonesia, Malawi, Nigeria, Tanzania, Uganda, Zambia, and… Read More
The Integration Readiness Assessment Tool developed by Clinton Health Access Initiative and partners helps countries gather situation analysis information regarding an individual facility’s readiness to provide integrated testing for tuberculosis (TB), HIV and HPV. The assessment output of this MS Excel-based checklist allows for identification of sites where TB/HIV/HCV/HPV integration on the GeneXpert platform would… Read More
The catalytic investment in point-of-care (POC), early-infant-diagnosis (EID), and viral-load (VL) technologies by the Accelerating Access to Innovative Point of Care HIV Diagnostics Project, implemented in partnership between UNICEF, Clinton Health Access Initiative (CHAI), ASLM, Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) and Unitaid, has shown significant results over the years. It has contributed to filling… Read More
On 18 February 2021, the LabCoP M&E Sub-Community of Practice held its second session that unpacked the fundamentals of monitoring and evaluation (M&E) and its importance in viral load (VL) monitoring. The use of VL data is essential for patient-level and program-level decision making. VL data is often unavailable, delayed, or underutilised, hindering monitoring interventions… Read More
On 11 February 2021, ASLM, in collaboration with the US Centers for Disease Control and Prevention’s International Laboratory Branch (CDC ILB) and partnership with Roche, hosted a webinar aimed at creating access to tools that will help laboratories improve their activities and services for viral load (VL) and early infant diagnosis (EID). The presentation provides… Read More
On 5 February 2021, ASLM’s LabCoP hosted the first ECHO session of the Monitoring and Evaluation (M&E) sub-community of practice. M&E assesses the performance of projects and programs set up by organizations to improve current and future outputs. A robust M&E system is critical to assess the performance of testing services and for decision making…. Read More
LabCoP’s January 2021 ECHO session covered PEPFAR’s Country Operational Plan 2021 and the laboratory systems strengthening priorities of the year. Dr George Alemnji, Senior Technical Advisor for PEPFAR Laboratory Services (OGAC), Washington DC, presented during the session. Please follow the links posted here to view the recorded video session on ASLM’s YouTube channel, and download… Read More
In November 2020 the LabCoP stakeholders met online to convene the 2020 Face-to-Face virtual meeting. LabCoP’s 4th Annual Meeting aimed to assess the progress of LabCoP countries’ action plans and the outcome of ongoing interventions; discuss laboratory system strengthening across diseases through reviewing challenges and best practices in maintaining routine viral load (VL), early infant… Read More
In this paper, Kenneth Kintu, et al, investigated the virological suppression (achieving viral loads < 50 copies per mL) before giving birth with dolutegravir compared to efavirenz, when initiated during the third trimester. They found that dolutegravir was associated with higher rates of viral load reduction in pregnant women compared with efavirenz. Although both regimens… Read More
In July of 2019, the World Health Organization and the African Society for Laboratory Medicine organized a meeting with countries and key stakeholders in diagnostics to discuss and find concrete ways to improve and increase access to integrated multiplex technologies and determine how they can be translated into public health policy that impacts global impact.
Laboratories need to conduct verification or validation studies to confirm that new tests for COVID-19 diagnosis perform as intended. In this document co-authored by ASLM, FIND and the London School for Hygiene and Tropical Medicine, further key requirements for quality assurance are defined, including quality control and external quality assessment that laboratories should follow to… Read More
LabCoP’s April ECHO session featured presentations about maintaining HIV and TB testing in the context of COVID-19 from Dr George Alemnji, Senior Technical Advisor for Laboratory Services at the State Department Office of Global AIDS Coordinator (SGAC) and Health Diplomacy, US CDC; Dr Lara Vojnov, Diagnostics Advisor, WHO; and Dr Dennis Falzon, Medical Officer, World… Read More
Mozambique provided this Excel-based tool, with its accompanying instructions, as a country example of a comprehensive diagnostic network optimisation or mapping tool at the July 2019 Global Diagnostics Integration meeting in Geneva. The government sought a tool that could be: subnational, consider multiple potential technologies as well as both laboratory-based and point-of-care, incorporate testing across… Read More
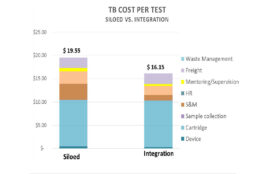
Developed by the Clinton Health Access Initiative (CHAI) for its country teams and ministries of health, this tool can be used to assess the financial benefits of integrating tuberculosis (TB), HIV, and HCV testing on the GeneXpert machine. The tool only covers cross-cutting costs (i.e. not disease-specific) regarding the use of GeneXpert, such as equipment,… Read More
The March 2020 waste management training session focused on Hologic’s Best Practices for Waste Handling. Todd Richmond of Hologic, shared his experiences, expertise and advice on the waste and contamination management of Hologic’s Panther. In collaboration with the African Society for Laboratory Medicine (ASLM) and the LabCoP community, the CDC International Laboratory Branch (ILB) offers… Read More
Pre-analytical challenges threaten the quality of viral load (VL) testing. This study determined the impact of delayed testing and warmer storage conditions on HIV RNA stability in diagnostic samples. Viral load in samples stored for up to a week reliably differentiated between patients with antiretroviral therapy (ART) suppression and ART failure in the majority of… Read More
Plasma preparation tubes (PPT) can simplify storage, transport, and preparation of plasma used for viral load (VL) testing. This systematic review evaluated the accuracy of PPTs for HIV VL testing. The results showed that following proper sample handling techniques helps provide accurate results. The authors thus recommended PPTs as a high-quality alternative specimen type for… Read More
This systematic review and meta-analysis summarizes evidence that shows that task shifting of testing using point-of-care technologies from laboratory professionals to non-laboratory staff was comparable to laboratory professionals operating the same technology in the laboratory. Task shifting of clinical tasks to lower cadres of health care workers and lay counselors has been successful in scaling… Read More
This paper by Jilian Sacks et al, 2019, presents a meta-analysis of studies comparing the Cepheid Xpert HIV-1 viral load (VL) plasma assay to traditional laboratory-based technologies. Cepheid Xpert HIV-1 VL plasma assay results were highly comparable to laboratory-based technologies with limited bias and high sensitivity and specificity to detect treatment failure. Alternative specimen types… Read More
This paper by Katherine Lamp et al, 2019, outlines an analysis of point-of-care (POC) CD4 invalid result rates across five countries. The authors found that the rate of invalid results was consistent across all types of health facilities, and the invalid rates were inversely correlated to operator usage, with high-volume operators. They concluded that POC… Read More
In this session, colleagues from Kenya shared findings from the assessment of waste handling practices in their viral load (VL) and Early infant diagnosis (EID) laboratories. They discussed contextual issues in regard to VL & EID laboratory testing capacity and networks, laboratory self-assessment using a VL & EID customized assessment checklist, findings/results, lessons learnt, challenges… Read More
These presentation slides are from the Women-Centered Diagnostics satellite session of the 2019 International Conference on AIDS and STIs in Africa (ICASA 2019) that was jointly organized by the African Society for Laboratory Medicine (ASLM), the Clinton Health Access Initiative (CHAI), the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF), UNICEF and Unitaid. Much has been done… Read More
To reach the third 90 of the UNAIDS 90–90–90 targets, country programmes must delve into their data and understand how they represent the quality of viral load (VL) testing services. This guide, published by WHO, presents key considerations and examples of tools (provided in the annexes) to assist countries in developing a national VL M&E… Read More
The November 2019 Waste Management training session focused on the perspectives of Abbott Laboratories’ Best Practices for Waste Handling. Delfin Rubin, Global Product Manager of HIV Care for Infectious Disease Emerging Markets at Abbott Rapid Diagnostics, and Ami Soni, EHS Lead for Abbott Molecular describe the constituents of their HIV rapid point-of-care diagnostics, such as… Read More
The purpose of this tool is to assist in identifying gaps and creating awareness of best practices for waste management processes in viral load (VL) and early infant diagnosis (EID) molecular testing laboratories (and associated healthcare facilities), in order to provide a starting point for assistance in waste mitigation strategies. This tool is for completion… Read More
In October 2019 the LabCoP stakeholders met in Addis Ababa, Ethiopia to convene the 2019 Face-to-Face meeting. It was co-convened by ASLM and the World Health Organization to enable countries to objectively assess their progress towards the improvement of the viral load testing (VLT) cascade, consolidate the implementation of interventions, enhance collaboration with civil society… Read More
Diagnostic network optimization is an exercise that aims to redesign the diagnostic network set-up in order to increase access, maximize impact, and generate efficiencies. It aligns testing demand and capacity in the most cost-effective way by defining the optimal instruments mix, identifying the most appropriate locations where instruments should be placed, and designing the referral… Read More
With limited funding for global health, identifying practical, cost and time-saving solutions, while also ensuring quality of care is increasingly important. One approach to increasing access to point-of-care (POC) testing is integrated testing (a term often used interchangeably with “multi-disease testing”), which tests for different conditions or diseases using the same diagnostic platform. This brief… Read More
Increased access to antiretroviral therapy (ART) and treatment monitoring for pregnant and breastfeeding women living with HIV is a priority for promoting health during the pregnancy and post-partum periods, and to minimize the risk of vertical transmission of HIV to infants. Point-of-care viral load (POC VL), which allows testing to be conducted at or near… Read More
This Waste Management session covered updates to the checklist for assessments of viral load (VL) and early infant diagnosis (EID) testing laboratories and associated healthcare facilities. David Bressler, MS, CBSP (SM) NRM of the International Laboratory Branch, CDC, Division of Global HIV and TB presented to the audience about the checklist/tool and its ability to… Read More
In the May 2019 session, Dr Katrina Sleeman, Associate Service Fellow in the Viral Load/Early Infant Diagnosis Team at the CDC ILB introduced a draft of a viral load WM checklist tool that can be used as baseline audit of select country VL labs. The tool aims to assist in creating awareness of best practices… Read More
In collaboration with ASLM and the LabCoP community, the CDC International Laboratory Branch (ILB) presented an overview of the World Health Organization (WHO) Publication: ‘Safe Management of Wastes from Health-Care Activities’ during the second ECHO session dedicated to waste management. This document, developed by WHO in 2017, highlights the key aspects of safe healthcare waste… Read More
In collaboration with the African Society for Laboratory Medicine (ASLM) and the LabCoP community, the CDC International Laboratory Branch (ILB) presented the first session in a series of ECHO sessions dedicated to waste management. In this session we reviewed waste management practices at the country level using the responses to the November 2018 LabCoP Waste… Read More
Several new laboratory technologies are available or are being developed to allow for testing of different conditions using disease-specific tests on the same platform. For example, a single device may be able to test for the presence of tuberculosis (TB) and HIV, and quantitatively measure HIV and hepatitis C viral load by using disease-specific reagents… Read More
This publication provides high-level guidance on implementing and scaling up HIV viral load (VL) testing programmes for health ministries and implementation partners, using a three-phased approach: (1) planning; (2) scale-up; and (3) sustainability. The guidelines for managing antiretroviral therapy (ART) issued by the World Health Organization have recognized the importance of viral load monitoring since… Read More
Broadens access to HIV viral load monitoring On July 20, 2017, Cepheid received World Health Organization (WHO) prequalification for its Xpert® HIV-1 Viral Load (VL) test. The Xpert® HIV-1 VL test measures Human Immunodeficiency Virus type 1 (HIV-1) RNA in human plasma from individuals infected with HIV-1 in less than 90 minutes. Measurement of blood… Read More
Representatives from the African Society for Laboratory Medicine (ASLM), UNICEF and the Clinton Health Access Initiative, Inc. (CHAI) came together 17-18 August 2017 in New York City, United States, to review progress to date on the implementation of a joint point-of-care project and to refine coordination mechanisms to achieve greater impact. “This project has… Read More